What Is the Formula for Beryllium and Nitrogen
To write the formula for Beryllium nitride well use the Periodic Table and foll. What is the formula of the compound that occurs when Strontium and Chloride combine ionically.

Solved Be Sure To Answer All Parts Give The Name And The Chegg Com
Ionic compound - 19969963.

. Beryllium is a hard grayish metal naturally found in mineral rocks coal soil and volcanic dust. It is a white to yellow powder with a density of 271 gcm 3. Beryllium nitride has the molecular formula of Be 3 N 2 and a molecular weight of 550652 gmol.
Notable gemstones high in beryllium include beryl aquamarine emerald and chrysoberylIt is a relatively rare element in the. Beryllium Nitride Properties Theoretical What is the ionic formula between Be and N. Its melting point is 2208C and a boiling point of.
For beryllium oxide the symbol Be is used for beryllium and the symbol O is used for oxygen. All samples of a given compound have the same proportions of their constituent elements. Nitrogen can form other ions such but nitride is the most common stable.
It can be prepared from the elements at high temperatures 1100 1500 C. Get an answer for What are the formula of ions formed by the following elements. Beryllium is a chemical element with the symbol Be and atomic number 4.
It is readily hydrolysed forming beryllium hydroxide and ammonia. It has two polymorphic forms cubic α-Be 3 N 2 with a defect anti-fluorite structure and hexagonal β. The chemical formula of any compound uses the symbols found on the periodic table of elements.
Beryllium and nitrogen. What is the formula of the compound that occurs when Beryllium and Nitrogen combine ionically. The chemical formula for beryllium oxide is BeO.
What is the formula of the compound that occurs when Aluminum and Oxygen combine ionically. Nitrogen is from Group 15 and can form nitride ions N3. What is the formula of the compound that occurs when Beryllium and Nitrogen combine ionically.
What is the chemical formula for beryllium and nitrogen. What is the empirical formula for nitrogen oxide. Write the empirical formula and draw Lewis dot structures for these ionic compounds.
BerylliumNitrogen Compounds 3. In this video well write the correct formula for Beryllium nitride Be3N2. The formula suggests a salt but as for many beryllium compounds the compound is highly covalent.
Be 3 N 2 19. When hydrolyzed in sodium hydroxide solution both nitrate and. Beryllium chloride is the name of the compound BeCl 2.
Matter cannot be either created or destroyed in a chemical reaction. Hydrogen Helium Lithium Beryllium Boron Nitrogen Oxygen. All atoms of a given element have a constant composition and are different than atoms of.
Additionally you wouldnt be expected to be able to predict the formula of other nitrogen ions Therefore the formula of the compound beryllium nitride is Be 3 N 2. What is the formula of the compound that occurs when Strontium and Chlorine combine ionically. Beryllium is element 4 with 2 valence electrons.
When added to water brown fumes are evolved. What is the formula of the compound that occurs when Lithium and Sulfur combine ionically. Little of its chemistry is well known.
Be3N2 decomposes in a vacuum into beryllium and nitrogen. It is readily hydrolysed forming beryllium hydroxide and ammonia. It is used in metallurgy as a hardening agent and in many outer space and nuclear applications.
Sodium chloride magnesium sulfide calcium fluoride potassium oxide beryllium phosphide strontium bromide barium nitride potassium iodide lithium bromide Page 2 of 2 3. Nitrous oxide commonly known as laughing gas or nitrous. The nucleus is a dense region of positive charge that always contains protons and neutrons.
Nitrogen has 5 outer shell electrons so will gain 3 electrons to form a nitride ion N 3-. Al 2 O 3 21. The salt that forms will be electrostatically neutral so a formula of Ba3N2.
It is a steel-gray strong lightweight and brittle alkaline earth metalIt is a divalent element that occurs naturally only in combination with other elements to form minerals. Beryllium nitrate is an inorganic compound with the idealized chemical formula Be NO 3 2. Nitrogen is element 7 with 5 valence electrons needing 3 to have a stable configuration.
Barium is an alkaline earth metal and forms Ba2 ions. Beryllium nitride Be 3 N 2 is a nitride of berylliumIt can be prepared from the elements at high temperature 11001500 C unlike Beryllium azide or BeN 6 it decomposes in vacuum into beryllium and nitrogen.

Click On Slide Show Click On From Current Slide Or From Beginning Ppt Download

Click On Slide Show Click On From Current Slide Or From Beginning Ppt Download

How To Write The Formula For Beryllium Nitride Youtube

How To Write The Formula For Beryllium Nitride Youtube
Solved Magnesium And Chlorine Express Your Answer As A Chegg Com

Unit 3 Every Element Wants To Be Happy Have Their Outer Energy Level Full An Element Can Accomplish This In One Of Two Ways 1 Electrons Can Be Ppt Download

Exceptions To The Octet Rule Chemistry For Non Majors

Solved 1 Use The Gizmo To Create Stable Compounds From The Chegg Com

Write The Symbol And Draw The Atomic Model For Carbon Hydrogen Nitrogen Magnesium Beryllium Oxygen Ppt Download

How To Write The Formula For Beryllium Nitrate Youtube
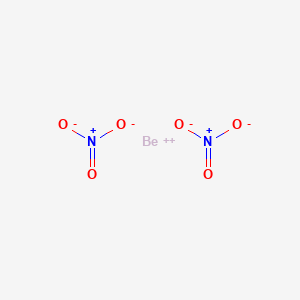
Beryllium Nitrate Be No3 2 Pubchem

How To Balance Ba N2 Ba3n2 Barium Nitrogen Gas Youtube

Solved Tonic Charges Chemical Formula Li A Lithium And Chegg Com

Review Game Unit 5 Element Bonding When The Highest Occupied Energy Level Of An Atom Is Filled With Electrons The Atom Is And Not Likely To Ppt Download

Ueq How Does The Structure Of Matter Influence Its Physical And Chemical Behavior Ppt Download
Chemical Nomenclature Mind Map

Ionic Bonding Periodic Table Quiz Quizizz

How To Draw The Lewis Dot Structure For Ba3n2 Barium Nitride Youtube

Click On Slide Show Click On From Current Slide Or From Beginning Ppt Download
Comments
Post a Comment